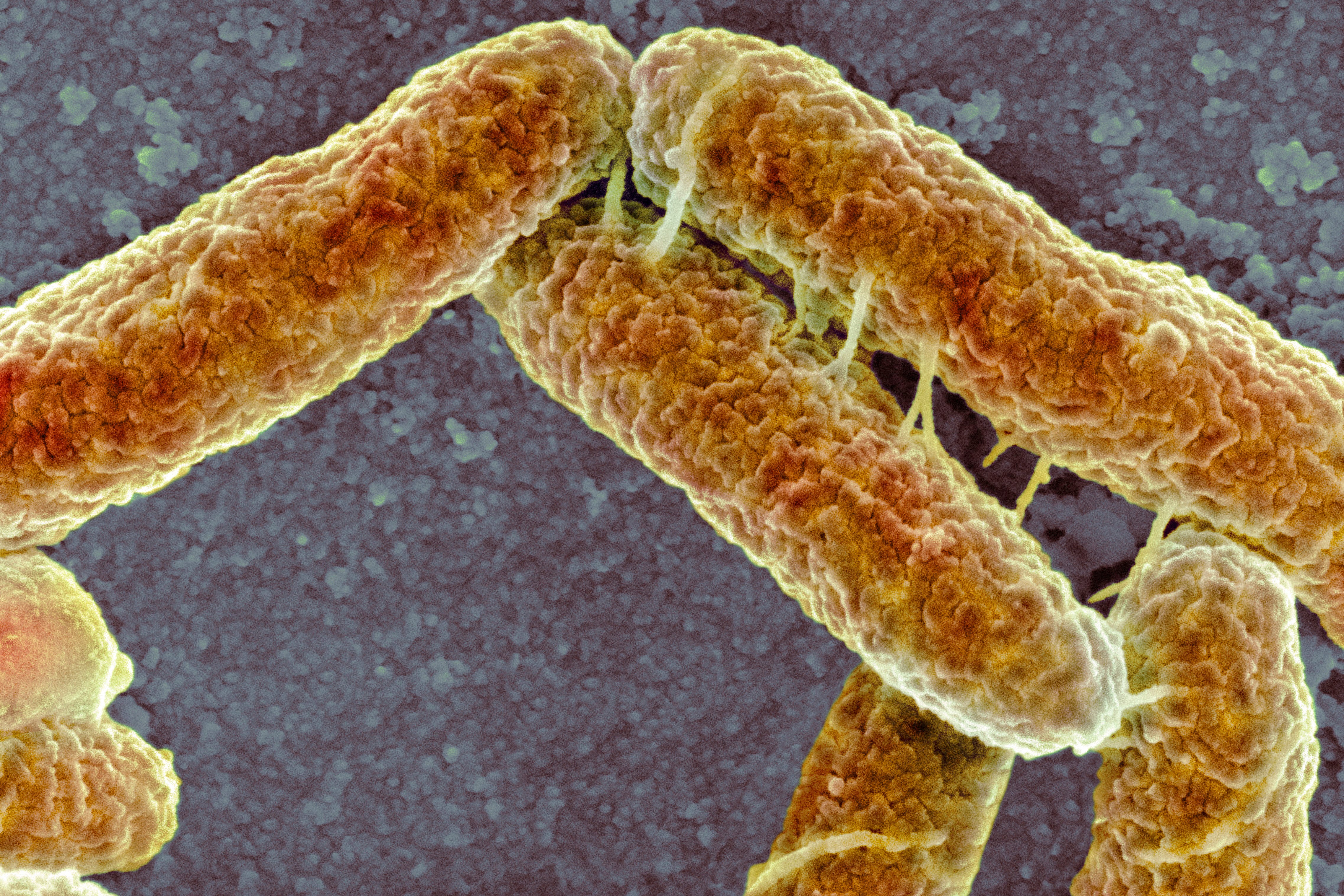
Bacteria may soon be muscling in on new kinds of manufacturing. Researchers have developed a technique that uses the common bacterium Escherichia coli to synthetically produce a muscle protein called titin, which could someday build tough and pliable fibers. Uses could range from medical sutures to impact-resistant or biodegradable fabrics. The titin is dozens of times larger than most molecules that have been produced in a laboratory, the researchers say.
Because E. coli is easy to control and replicates quickly, scientists use it to produce many kinds of substances, including biodiesels and pharmaceuticals. Until recently, however, synthesizing bigger proteins such as titin—which is about 50 times the size of the proteins E. coli naturally makes—has been out of reach.
In a new study detailed in Nature Communications, the researchers spurred E. coli to manufacture titin by introducing a circular strand of engineered DNA instructions called a plasmid. But building such a large protein drains cellular resources, says study co-author and Washington University in St. Louis biochemist Cameron Sargent. If a plasmid instructs E. coli to build the whole protein at once, the bacterium will avoid the production burden by removing or truncating the plasmid. So the team instead engineered a plasmid that makes E. coli construct shorter protein fragments that are structured to spontaneously link together inside the bacterium.
Once scientists extracted titin from the bacteria, they dissolved the proteins at high concentration in an organic solvent. Next, they squirted the solution through a syringe into a water bath, letting the proteins assemble along an emerging fiber as it was spun—an extrusion process inspired by how spiders build silk draglines. The engineered strands’ strength and toughness exceeded that of natural titin as measured in muscle fibers.
Sargent likens titin molecules’ size and arrangement within the fibers to a pot of congealed spaghetti. “It’s a lot harder to pull long spaghetti apart compared with short spaghetti because the longer the spaghetti is, the more interactions there are between each strand,” Sargent says. The researchers found that the way these interactions react to stress is key to titin’s toughness: when the fibers are pulled on, bonds within the molecules break first, absorbing much of the applied force’s energy before the bonds between strands eventually snap.
Inha University chemical engineer Yun Jung Yang, who was not involved in the study, says she finds it “remarkable that the researchers were able to replicate the actual mechanical properties of natural titin in an engineered protein.”
Researchers can now apply this manufacturing technique to other large proteins, making it feasible to explore additional candidate biomaterials—such as resilin, an elastic polymer that powers a flea’s jump, or the tough mother-of-pearl that lines abalone shells—for practical use.
