On January 19, co-founders Rick Klausner and Hans Bishop publicly launched an aging research initiative called Altos Labs, with $3 billion in initial financing from backers including tech investor Yuri Milner and Amazon founder Jeff Bezos. This is the latest in a recent surge of investment in ventures seeking to build anti-aging interventions on the back of basic research into epigenetic reprogramming (modifying chemical marks on DNA to turn genes on or off). In December, cryptocurrency company Coinbase’s cofounder Brian Armstrong and venture capitalist Blake Byers founded NewLimit, an aging-focused biotech backed by an initial $105 million investment, with the University of California, San Francisco’s Alex Marson and Stanford’s Mark Davis as advisors.
The discovery of the ‘Yamanaka factors’—four transcription factors (Oct3/4, Sox2, c-Myc and Klf4), ), proteins that can reprogram a fully mature cell into an embryonic-like state—earned Kyoto University researcher Shinya Yamanaka a share of the Nobel prize in 2012. The finding, described in 2006, transformed stem cell research by providing a new source of cells that resemble embryonic stem cells, which are able to give rise to any type of specialized cell in the body except sex cells. These induced pluripotent stem cells (iPSCs) do not require human embryos for their derivation. But in recent years, Yamanaka factors have also become the focus for another burgeoning area: to set back the clock on aging.
So-called partial reprogramming consists of applying Yamanaka factors to cells for long enough to roll back cellular aging and repair tissues but without returning to pluripotency in which a cell can specialize into other cell types. Several groups, including those headed by Stanford University’s Vittorio Sebastiano, the Salk Institute’s Juan Carlos Izpisúa Belmonte and Harvard Medical School’s David Sinclair (See Table), have shown that partial reprogramming can dramatically reverse age-related characteristics in the eye, muscle and other tissues in cultured mammalian cells and even rodent models by countering epigenetic changes associated with aging. These results have spurred interest in translating insights from animal models into anti-aging interventions. “This is a pursuit that has now become a race,” says Daniel Ives, CEO and founder of Cambridge, UK-based Shift Bioscience.
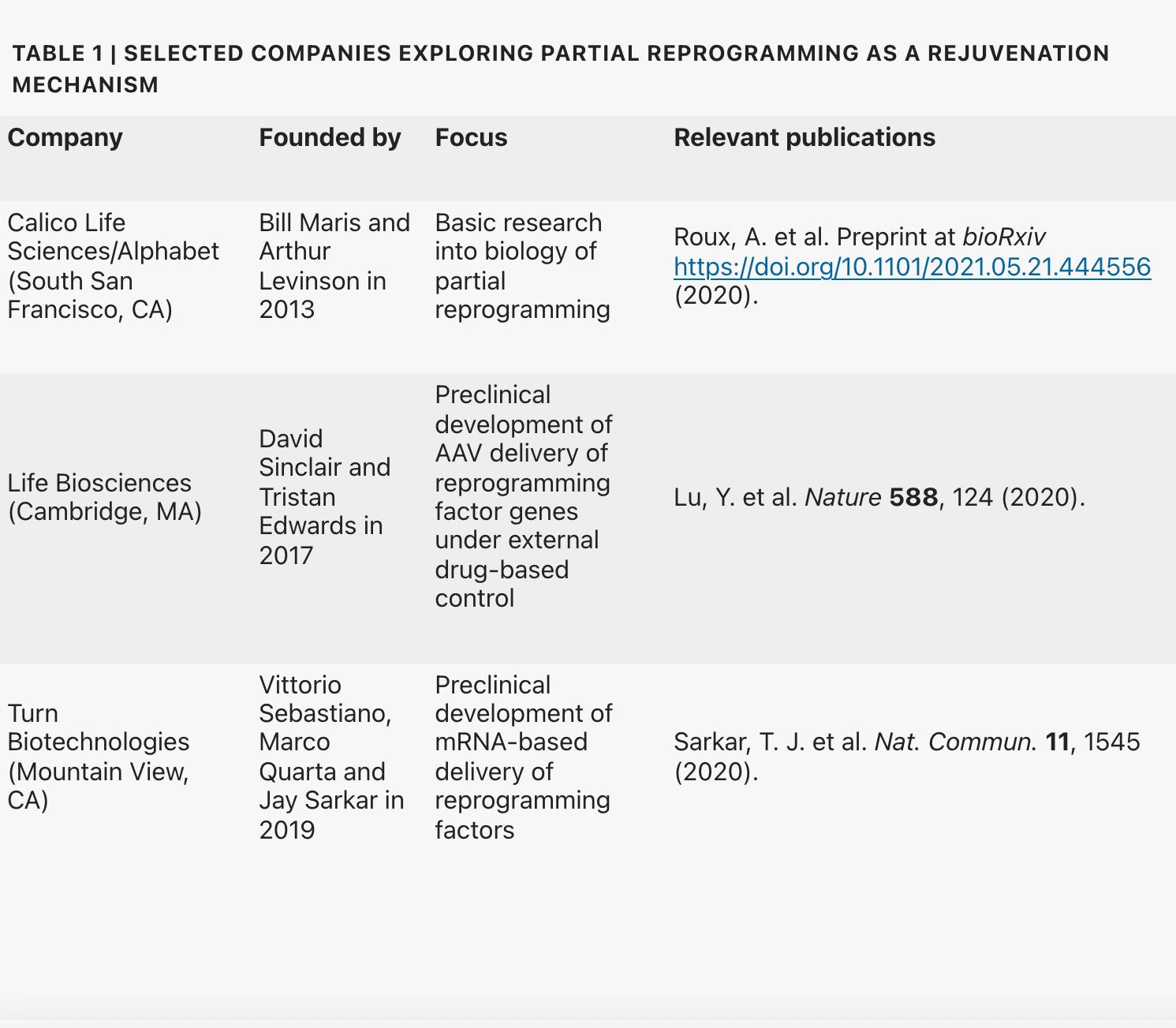
“We’re investing in this area [because] it is one of the few interventions we know of that can restore youthful function in a diverse set of cell types,” explains Jacob Kimmel, a principal investigator at Alphabet subsidiary Calico Life Sciences in South San Francisco, California. The zeal is shared by Joan Mannick, head of R&D at Life Biosciences, who says partial reprogramming could be potentially “transformative” when it comes to treating or even preventing age-related diseases. Life Biosciences, a startup co-founded by David Sinclair, is exploring the regenerative capacity of three Yamanaka factors (Oct4, Sox2 and Klf4).
Even though Life Biosciences and several other startups are investigating Yamanaka factors with a view to reversing human aging, the biology of rejuvenation by reprogramming remains enigmatic and opaque, at best. “These first papers make some astonishing observations,” says Konrad Hochedlinger of the Harvard Stem Cell Institute. “But much more research is needed to dig into the molecular and mechanistic processes that are occurring.” Given that fully reprogrammed iPSCs readily form tumors known as teratomas, scientists must determine whether the cellular clock can be wound back safely in humans—which means the race to the clinic will likely be a marathon rather than a sprint.
Yamanaka’s technique, which can even generate biologically youthful stem cells from centenarian donors, has been extensively studied over the past 15 years. Alexander Meissner of the Max Planck Institute for Molecular Genetics says that most iPSC reprogramming comes down to rewriting epigenetic marks—chemical modifications of the genome, such as adding methyl groups to DNA or to the histone proteins that act as packaging for DNA. This process influences which genes are activated and which tend to change as cells age. “You erase all of these signatures that look like aging or any abnormal signatures, and [cells are] being reset essentially to the baseline ‘perfect’ epigenome,” Meissner says.
This reprogramming reconfigures gene expression networks that switch genes on and off, thus reversing age-related cellular features. But several researchers have hypothesized that partial reprogramming could potentially make cells younger without pushing cells all the way back to an undifferentiated, embryonic state, diminishing the prospect of a tumor.
In 2016, Izpisúa Belmonte and colleagues genetically modified a mouse model of progeria—a premature aging condition caused by mutated lamin proteins that make up cellular support structures. They did so to express the four Yamanaka factors. His team regulated these factors’ expression by treating animals with the drug doxycycline, which had been programmed in as a regulatory ‘switch’ to control gene expression. This conditional switch proved essential, because when the factors were continuously expressed, the mice died within days as a result of organ failure. But by activating the factors at brief intervals, the researchers were able to extend life expectancy for the progeroid mice and restored youthful function in multiple organs.
To estimate a tissue or a cell’s age, Steve Horvath at the University of California, Los Angeles, developed an ‘epigenetic clock’—a means of estimating biological aging using DNA methylation from different cells and tissues. In 2019 The University of Edinburgh’s Tamir Chandra and team showed that partial reprogramming can turn back this clock before cells ultimately lose their identity. But Chandra cautions that “I’m not convinced that there is causality,” and that the clock may be a readout for a broader process of cellular rejuvenation. Meissner likewise notes that many of these epigenetic changes seem to be located far from known genes or regulatory elements.
The partial reprogramming experiments proved promising and spurred other academic groups to pursue a similar approach in different animal models of aging. In 2020, Sinclair’s team reset the epigenome to restore vision in mice using the adeno-associated virus (AAV) vector to deliver three Yamanaka genes—they excluded c-Myc because of its known oncogenic properties. The expression of the transcription factors in retinal ganglion cells reversed vision loss in mice, promoting axon regeneration after optic nerve injuries or glaucoma in aged mice. Critically, the researchers saw no sign of lost cellular identity, even when the three Yamanaka genes were continuously expressed.
Could the same happen in human cells? Sebastiano’s team showed that mRNA-based expression of the four Yamanaka factors plus two accessory factors (LIN28 and NANOG) to boost reprogramming efficiency could reverse epigenetic and inflammatory signatures and restore regenerative potential in a range of cell types from aged people—cultured fibroblasts, endothelial cells and chondrocytes. “We have seen this now across almost 20 different human cell types,” Sebastiano says.
Multiple companies have sprung up to build on these pioneering studies: Sebastiano co-founded Turn Biotechnologies, Sinclair set up Life Biosciences, and Izpisúa Belmonte is reportedly joining Altos Labs. Calico’s Kimmel, as well as other startups, including Shift Bioscience, Retro Biosciences, YouthBio Therapeutics and AgeX (initially a subsidiary of Michael West’s BioTime), are likewise investigating the rejuvenating potential of partial reprogramming.
But the route to safe and effective human aging interventions is unlikely to proceed via systemic delivery of Yamanaka factors. Direct injection would be far too risky due to the chances of inducing malignancies resulting from turning back cells to their embryonic, dedifferentiated state. “It would be very difficult to de-risk this sufficiently,” says Kimmel.
Even the iPSC experts are unclear as to when reprogrammed cells reach the ‘point of no return’ and revert to a fully embryonic-like state. In Hochedlinger’s experience, the timing “can range from a week to only two or three days after reprogramming.” A recent non-peer-reviewed preprint by Kimmel and Calico’s VP of Aging Research Cynthia Kenyon further highlights the uncertainty of this process, showing that even transient Yamanaka factor activation can cause gene expression changes in human cells that indicate a loss of cellular identity. This isn’t simply an academic question—the fundamental safety of this approach hinges on the answer. Indeed, the formation of tumors called teratomas is a common metric for assessing cellular pluripotency in reprogrammed iPSCs. “Once a single cell has made it over to an iPSC, that single cell is sufficient to make a tumor,” says Hochedlinger.
It remains unclear whether an optimal combination of Yamanaka factors introduced at just the right time can fully mitigate the risk of teratomas. Mannick says that mice treated by the Sinclair group with the three Yamanaka factors other than c-Myc have remained tumor-free for nearly a year and a half since treatment. But translation of this research into humans will require setting a much higher bar. “Safety is the most important thing that we’re dealing with right now,” says Sebastiano.
To minimize the risk of reprogramming cells to a pluripotent state, Life Biosciences is focusing initially on a single, well-studied tissue: the eye. Mannick says that the company’s preclinical work indicates that the eye offers a safer starting point than other tissues because few cells proliferate in that tissue.
Turn Biotechnologies is focusing instead on the skin, where Sebastiano sees potential in cosmetic indications as well as more serious age-related issues like impaired wound healing. He adds that the accessibility and detailed understanding of the skin can also help accelerate translation. Turn uses a lipid-nanoparticle-based delivery of mRNAs to make the Sebastiano group’s cocktail of six reprogramming factors: Oct4, Sox2, Klf4, Oct-4, LIN28 and NANOG. Although these factors cannot be externally regulated, the short half-life of Turn’s mRNAs should limit their activity to a few days. Another option Turn is pursuing is to reprogram cells outside the body, which would allow the lab to perform quality control on cells before returning them to patients.
Leaving c-Myc out of the reprogramming mix may be advantageous, according to Hochedlinger. He stresses, however, that two other Yamanaka factors, Sox2 and Oct4, in Life Bioscience’s cocktail have also been linked to cancer. Tumors could remain a risk even for cells that maintain their identity. Aged cells typically acquire many mutations over their lifetimes; Chandra notes that these are not erased by rejuvenation, and some may have been selected for because they confer an edge for proliferation or survival. “They are already, in a way, one step towards cancer,” he says. “So what happens if they see Yamanaka factors?”
Some companies are already looking beyond Yamanaka factors. Shift Bioscience is looking for workarounds that use machine learning to identify genes that help reverse biological aging but that do not contribute to pluripotency. “We basically think we’ve got line of sight on the rejuvenation pathway during cellular reprogramming,” says Ives. “These genes look safe, and they don’t look like they’re going to affect cell identity.” He adds that Shift is still fleshing out these rejuvenation-associated gene networks and will then move to test the safety and efficacy of different combinations of these genes in a range of cell types.
Indeed, the Yamanaka factors may be most valuable as a tool for studying basic biology of aging and rejuvenation rather than as therapy. Meissner says: “I doubt it’s a good idea to induce these pluripotency factors in any individual.” Kimmel likewise sees limited clinical applications for current reprogramming approaches, adding that Calico is primarily pursuing this work to explore fundamental questions about aging. “Right now, this is not something where we’re thinking clinically,” he says.
With so many unanswered questions, companies in this space need to prepare for a long-term investment. Deep-pocketed firms with billionaire backing—like Altos, which is setting up institutes in the San Francisco Bay Area; San Diego; and Cambridge, UK—may have an edge here. Chandra says that colleagues who have signed on with the firm “are not being pressured into delivering anything, except for good science for the first five to ten years.”
Above all, those pursuing rejuvenation therapeutics know about the need to manage expectations. “This message needs to be clear—it’s not about extending the lifespan,” says Sebastiano. “What we care about is increasing the healthspan of people … and that you don’t have to live for a long time in a condition of frailty.”
This article is reproduced with permission and was first published on January 19 2021.
